<< Back to Copper Recycling and Sustainability Part 2 (16-18)
End of Life Vehicles (ELV)
About 2 million vehicles are scrapped each year in the UK and this represents a huge volume of waste and a potential source of material for reclamation by recycling. An EU Directive (2007) will help to ensure that more vehicles are recycled when they are scrapped.
Copper in cars and lorries
The incorporation of electronics and electrically powered accessories has raised the length of copper wiring in a family car to about a kilometre, from an average of around 45 metres fifty years ago. The starter motor, lights, electric windows, anti-lock braking systems (ABS), air bags, entertainment systems and satellite navigation all rely on the high electrical conductivity of copper.
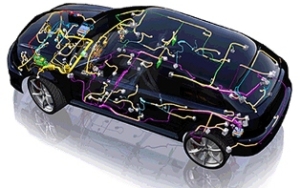
A modern car has more than a kilometre of copper cable for sensors, switches, safety systems, power systems for lamps, air conditioning, brakes and electronic data communication. These cables are hard to get out of a scrap car. Motor windings and alternator windings are easier to remove. (Courtesy of Delphi.)
Copper and copper alloys have a unique combination of properties, which make them the first choice for a wide range of applications in cars and lorries. The weight of copper in a vehicle ranges from 15 kilos for a small car to 28 kilos for a luxury car and the benefits copper brings are illustrated in the applications shown below.
Properties and Applications
High electrical conductivity
Copper has the highest electrical conductivity of any commercial metal and so copper wires (called the wiring harness) are used to carry current from the battery to equipment such as lights, central locking, on-board computers, in-car entertainment systems, satellite navigation and safety features such as airbags and ABS braking systems. Electric motors, which are wound with high conductivity copper wire, are used in many of these devices. The average car contains about 1km of wire.
High thermal conductivity
When cars are driven, the engines become very hot. This heat is removed with the aid of a coolant liquid, which passes through a radiator that must be made from a material with high thermal conductivity and good corrosion resistance. Copper and brass are excellent choices for this application. Radiators are removed from vehicles at the end of a car’s life for recycling. The thermal conductivity of copper is 30 times better than the thermal conductivity of stainless steel.
Good corrosion resistance
Copper and copper nickel tubes are used for vehicle brake pipes, which are critical safety items. This is due to their good strength and corrosion resistance (especially against salt spread on the roads in winter), where any fluid loss could lead to brake failure and be disastrous. Because of their ductility they are also easy to fit; they are standard equipment for many cars, fire engines, military vehicles, JCBs and other heavy vehicles.
Good bearing properties
In contact with moving parts in the engine, copper alloys provide a surface which does not stick or wear easily whilst being strong enough to provide support. In this application it acts as a bearing.
End of life vehicles (ELV)
An ELV is any vehicle that has come to the end of its useful life.
ELV Directive
About 2 million vehicles are scrapped each year in the UK, and the European ELV Directive, which came into force in January 2007, is an attempt to minimise the amount of waste generated from ELV disposal. Sites which deal with ELV have to be licensed and meet high environmental standards. A typical ELV disposal site can deal with 180 cars per hour and the stages are:
- Dismantling: any valuable parts are removed
- De-pollution: all fluids such as oil and petrol/diesel are removed and recycled
- Removal of wheels, tyres and battery
- Shredding: the vehicle carcass is then smashed to small pieces with giant hammers spinning at 500 rpm
- Vacuum treatment: the light non-metals (foam, fabric and wood) are sucked up and currently sent to landfill
- Magnetic treatment to remove steel
- Density separation of copper: the higher density of copper (8.9 g/cm3) enables it to be separated from aluminium (2.7 g/cm3) and magnesium (1.7 g/cm3).
This is the only economical way to recover copper (and other metals). It is not feasible to remove components such as the wiring harness in one piece by hand; it would be too time-consuming and expensive. About 80% of the copper is able to be recovered by this process; this value should increase as techniques improve.
Recycling Through History
Bronze is an alloy of copper and tin, which is harder and stronger than copper. Bronze is easier to cast than copper and in ancient times useful objects such as axes, knife blades, swords and helmets were produced, as well as decorative items such as bells, buckles, drinking vessels and statuettes. The earliest bronzes are dated 4000 BC.
Bronze is so important that periods in history were named The Bronze Age. It is likely that bronze was discovered independently in many areas of the world so that there is not one Bronze Age which applies to all areas. The earliest Bronze Age may be 3500-2000 BC in what is now Turkey, Syria and Iraq, and 2100 to 700 BC in Britain. In these early days, Cornwall was highly industrialised and a most important area for mining tin and copper and soon became a major exporter of these metals with well established trade routes to Mediterranean countries. No tin or copper is mined now in Cornwall (or anywhere else in the UK). Only a few ruins and many deep holes in the countryside remain.
Bronze is very suitable for making bells and bell founding and the making of large bells was one of the earliest metal industries in Britain.
The Whitechapel Bell Foundry in London is listed as Britain’s oldest manufacturing company, established in 1570 (during the reign of Elizabeth I) and in continuous business since that date. The most famous bell made in Whitechapel is Big Ben, which was cast in 1858 weighing 13.76 tonnes. The manufacture of Big Ben used recycled bronze obtained from an earlier bell (not made at Whitechapel), which had cracked on testing. The original quoted price of £2,401 for Big Ben was offset by an allowance of £1,289 for the scrap bell making a final cost of only £572. This is an early example of the value of recycling and sustainability.

The technology of bell founding was extended to cast bronze cannon as long ago as the 14th century. These bronze guns often allow the identification of archaeological sites. As bronze corrodes only very slowly, these guns often retain the maker’s name or the owner’s name and the date. Henry VIII had a flagship called ‘The Mary Rose’ which sank during a naval review in 1545. It has yielded many bronze and brass cannon, which confirm the identity of the wreck.
Bronze medals and medallions can look like coins but are not used as currency. The first English service medal was the Armada medal awarded in 1588 by Elizabeth I.
Bronze plaques on buildings commemorate famous people and events. Bronze is chosen because it is easy to cast, reproduces fine detail and resists corrosion.
The Victoria Cross (VC) is the highest military decoration for valour. It was instituted in 1856 by Queen Victoria. Medals were originally made from bronze recycled from two Chinese-made cannon captured from the Russians at Sebastopol during the Crimean War (1854-56). The gunmetal casting contained 10% tin.

Bronze and Recycling
Historically, bronzes were used for their hardness, strength and easy castability and gave their name to a period of time known as The Bronze Age. However, bronze is not simply an alloy of the past; it is very much used in modern engineering applications.
Ships
Ancient bronzes consisted mainly of copper and tin and are called tin bronzes. Tin bronzes are still used today but bronzes containing aluminium, nickel, manganese and silicon have been developed to give increased strength and corrosion resistance. These bronzes are used in seawater applications and are fully tested to meet stringent government requirements.
An example of the use in seawater is the propellor of a container ship (see image). This was sand cast in nickel aluminium bronze and weighed 95 tonnes. It was produced in one continuous pour and was fed with liquid bronze for 24 hours, since during solidification the bronze contracted.

95 tonne aluminium bronze propellor for a container ship. (Courtesy of Stone Manganese Marine Ltd.)
It was made from almost 100% scrap which was sourced from in-house process scrap from other projects, together with carefully controlled bought in scrap.
It is clear that over the centuries bronze has been recycled to good effect, another example of the sustainability of copper alloys.
Architecture
Architects also use bronze widely to enhance the appearance of their buildings, taking advantage of its attractive colour, corrosion resistance and high strength. A good example of this is Portcullis House, the parliamentary building, which is clad in aluminium bronze plate.

Portcullis House has an aluminium bronze roof and cladding panels. (Creative Commons.)
Statues
Thousands of years ago bronze was used to produce iconic statues such as the Colossus of Rhodes. This was destroyed by an earthquake in 224 BC and the pieces were sold. The scrap bronze needed 900 camels to carry it away for recycling.
Bronze continues to be used as the first choice for modern statues; recent examples being those of Bobby Moore (outside Wembley Stadium), Nelson Mandela (in Parliament Square) and ‘The Meeting Place’ (in St Pancras International Station, London).

Bronze statue of Bobby Moore outside Wembley Stadium, London (Creative Commons).
These bronzes have similar compositions to the ancient bronzes, being alloys of copper, tin, lead and zinc. Discarded old bronze castings can be remelted and this recycled material used to make new statues. Statues typically contain 30% recycled material. No bronze is thrown away; it is always reused just as it was in 224 BC.
Nuclear Submarine Case Study
An example of large scale recycling of metals (including copper/copper alloys) is described following the dismantling of the Russian Oscar 1 class cruise missile submarine.

Dismantling a Russian nuclear submarine. (Courtesy of Keel Marine.)
About Oscar
The submarine was built in 1980–82 and was in service from 1983–1997 and dismantled between 2003 and 2004. It was 345 metres long, 18 metres wide, carried 24 cruise missiles with 6 bow torpedo tubes. It had a displacement of 17,000 tonnes when submerged and carried a crew of 107.
Dismantling
An international agreement was reached to dismantle the submarine and, as part of this effort, a UK company, Keel Marine Ltd, provided marine expertise and support during the dismantling process.
The dismantling of a nuclear submarine is clearly a complex process. Although the spent nuclear fuel was removed before dismantling started, care had to be taken to remove toxic waste such as rubber acoustic tiles, lubricants, oils, glass, refrigerants, insulation and asbestos. Working temperatures of -30 degrees centigrade were not uncommon.
- Metals and alloys recovered for recycling
Steel plate, used to construct the hull, from 10 to 40mm thick: 6,000 tonnes - Titanium – this is a lightweight and corrosion-resistant alloy: 600 tonnes.
- Copper alloys – tin bronze and aluminium bronze: 180 tonnes.
- Components on the submarine such as pumps, pipes, valves and taps that were exposed to seawater, which causes metals to corrode, were made from these corrosion resistant copper alloys.
- High purity copper wire: 72 tonnes.
The submarine had many kilometres of high purity electrical copper cable, vital for the communication, navigation and weapon systems and lighting. Oxygen and fresh water are both generated by electrical means, a vital facility when the submarine was submerged for long periods.
Copper granules
The insulation was stripped from the cable and the copper wire chopped into small pieces (about the size of coffee granules). About 72 tonnes of high purity copper was recovered, which could be remelted and made into new copper wire without the need for any refining (purification). This clearly is a very valuable product and shows once again the sustainable nature of copper. It is interesting to speculate where this recycled copper would appear next, perhaps as part of your next mobile phone or computer.

Granules of copper formed after chopping up the kilometres of copper cabling.
1. In Chart 2 (below), why is the ratio of tonnage of primary and secondary sources not the same as the 59% to 41% of sources of copper?
2. Why is bronze used to make ship propellors? (Think about manufacturing and use at sea.)
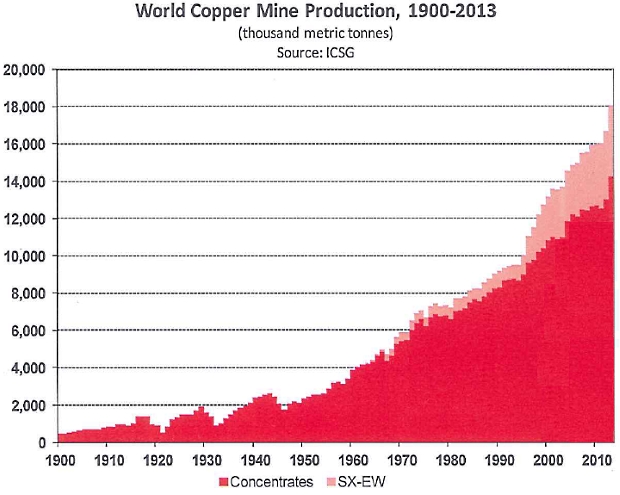
3. In Chart 1 (below), which form of copper extraction has grown from zero in the 1960s to almost 4 million tonnes in 2013?
 |
 |
Comments
0 comments
Please sign in to leave a comment.