Pure copper (99.9%) is the best electrical conductor apart from silver. Imagine a world without electricity; no computers, no tablets or mobile phones, no house or street lighting and no electrified railways. You can probably think of other examples.
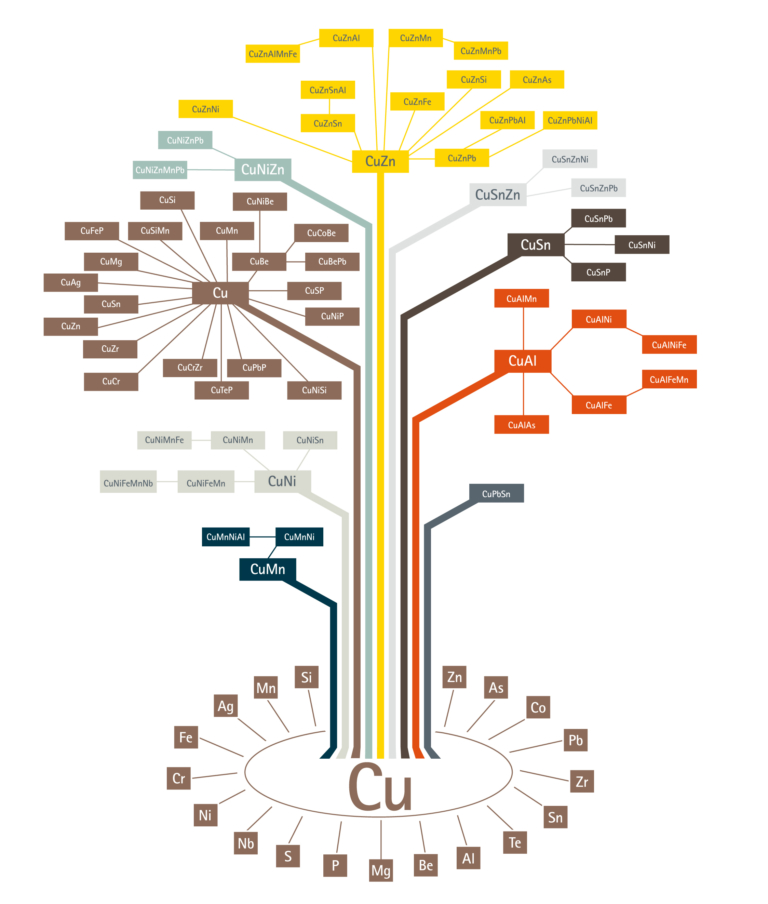
Pure copper is, however, very soft and low in strength, and tiny additions of alloying elements such as silver, tin, iron and phosphorus are made to improve the hardness and strength of copper without lowering the electrical conductivity too much. An example of this is a copper-silver alloy used for overhead conductor wires on electrified railways.
The history of alloys goes back thousands of years. Long before electricity was invented, ancient man discovered that copper alloyed with tin made the very first useful alloy, bronze. Bronze is much harder and stronger than copper and much tougher (less brittle) than flint or stone. The properties could be further improved by hammering (known as work hardening). This development was so important that periods in history were named the Bronze Age. In Britain, this was 700 to 210 BC.
Man gradually moved from the stone to the metal age. Much later than bronze came brass (copper and zinc), which offered improved hardness, strength and ductility in a range of attractive colours from red to yellow to white (like stainless steel).
In more modern times, copper has been alloyed with nickel (making cupronickels) to give a range of alloys that are used in the most severe seawater conditions, such as those found on oil rigs.
The strongest copper alloys are copper with beryllium. These can be made as strong as steel, but are reserved for special applications since they are relatively expensive.
Remember that all of the above alloys can be remelted and reused. This is called recycling and is one property which makes all copper alloys sustainable.
The four sections below look at a few specific applications of copper alloys in more depth.
- Copper Alloys in Aquaculture (14–16)
- Copper Alloys in Coinage (all ages)
- Copper Alloys in Music (14–16)
- Copper Alloys in Sculpture (all ages)
Do you want to read more about copper alloys and their uses in industry? Click here to begin exploring Copper Development Association’s dedicated alloys section.

Comments
0 comments
Please sign in to leave a comment.