Copper is one of the least reactive metals. However, it does react with a solution of ferric chloride and also copper chloride. This has been known for a long time and is a common way of etching copper.
Etching is a basic tool to reveal the grain structure of copper. The etchant attacks the polished copper surface selectively revealing the size and shape of the grains.

(Courtesy of Copperheart.)
The flower patterns on these jewellery discs were painted with a lacquer that resists ferric chloride. The exposed areas have been partly dissolved by the ferric chloride, leaving a raised pattern.
For the 2012 London Olympics, Thomas Heatherwick Studio designed the now world famous cauldron made from copper petals. Each one had the name of the competing country etched into the copper with ferric chloride solution.

The letters on this copper petal were etched with ferric chloride solution. (Courtesy of Heatherwick Studio.)

A masking tape protects the copper from the ferric chloride solution except where the letters are. (Courtesy of Heatherwick Studio.)
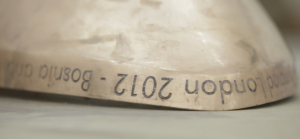
The petal for Bosnia and Herzegovina before polishing… (Courtesy of Heatherwick Studio.)

…and after. (Courtesy of Heatherwick Studio.)
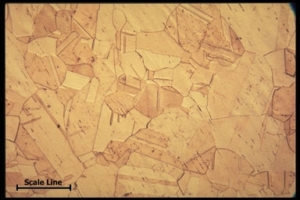
The grain structure of a metal determines the properties such as strength and ductility. The image above is magnified 200 times and shows the grain structure of etched copper.
The Chemistry of Copper Etching
The process has three reactions:
Equation 1 FeCl3 + Cu → FeCl2 + CuCl
Equation 1 will do the etching by itself because the metallic copper atom has been oxidised and becomes a copper ion within the copper chloride in solution, but a second reaction happens, making this a two-step redox reaction.
Equation 2 FeCl3 + CuCl → FeCl2 + CuCl2
The copper(I) chloride is further oxidised to copper(II) chloride
Just to complicate matters, the copper(II) chloride that builds up in the etching solution because of equation 2 also reacts with copper. So now we have two etchants working although we started with just one.
Equation 3 CuCl2 + Cu → 2CuCl
Mass Producing Circuit Boards
Etching can only be used for prototype boards and for school or hobby electronics. It is difficult to control the process and very hard to make narrow tracks.
Commercial boards use copper electroplating. A circuit board, with only a very thin copper layer, is made to become the cathode (negative) plate in an electroplating tank. Copper is then deposited on the tracks and builds up until they are thick enough. This process is the reverse of etching. To find out more, try searching for ‘semi-additive process’.

(Wikimedia/Innoquick.
Circuit boards in an electroplating machine. Cu2+ ions are attracted to the board and deposited as copper metal. The blue areas do not get plated as they are coated in a film called a resist.
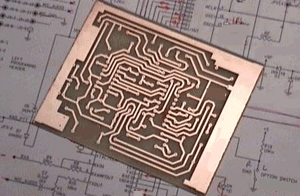
(Courtesy of QSL.)
Ferric chloride was used to etch away the copper on this board, leaving only the circuit tracks remaining. The tracks were covered with a protective resist layer to protect the copper. Areas with no resist have lost all the copper, leaving the fibreglass board exposed. Mass produced boards are not made this way. They are made by electroplating copper onto the board to make the tracks.
Questions
1. Copper atoms in a metal have no charge, but copper ions in solution as copper chloride have a positive charge. What has the copper atom lost to change it into a positive ion?
2. In the electroplating process copper ions in solution are deposited on the tracks, which are connected to an electrical negative terminal to make them the cathode. Complete this electron half equation for what happens at the cathode:
2+(aq) + 2e- → ?
 |
 |
Comments
0 comments
Please sign in to leave a comment.