<< Back to Copper Mining and Extraction: Oxide Ores (14-16)
1. Why can the electrolytic refining (electrowinning) be done without the use of copper anodes?
In electrolytic refining using copper anodes, the copper is in the anode. In electrowinning the copper is in the electrolyte.
2. In electrolytic refining of anode copper from sulfide ores, small amounts of impurities from the anode form a sludge containing precious metals. In SX-EW there is no sludge. Explain why.
In electrowinning there is no copper anode. The electrolyte contains copper ions with minimal impurities. The precious metals are left in the leach pad and not recovered.
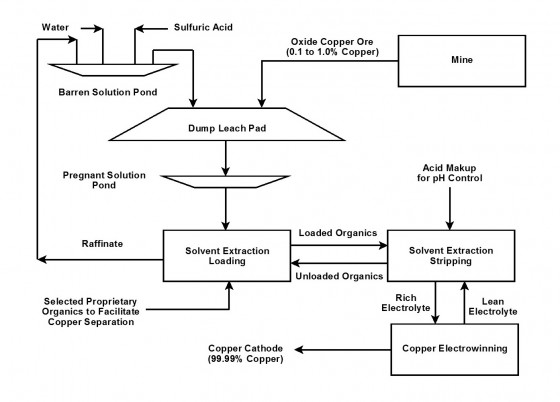
This chart shows how sulfuric acid is used to extract copper. The main difference between this and the ‘pyroprocessing’ of sulfide ores is that no heat is used.
Comments
0 comments
Please sign in to leave a comment.