Copper plays a vital role in shaping our lives. In this collection of resources, you can find out about the biology, chemistry and physics of copper.
The Roles of Copper
In the home
Copper is hidden away in everyday objects around our homes, including phones, water pipes, locks and electrical wiring.
In history
People have been using copper since 9,000 BC. One of the reasons copper is so important is that it can be made into alloys. That means it can be combined with other metals to make new alloys, like brass and bronze. These are harder, stronger and more corrosion resistant than pure copper.
For its properties
Copper is an excellent conductor of electricity and heat; it is strong, ductile and easily joined by soldering or brazing; and it is hygienic, easy to alloy and resists corrosion.
Pure copper has a list of properties that make it vital to modern technology.
Some Important Properties of Copper
Electrical conductivity
When a voltage from a battery is placed in the circuit in Figure1 an electric current will flow. An electric current is a flow of electrons. In a metal there are electrons that are not attached to atoms. They are called free electrons. They are free to move between the atoms. It is not the battery that frees them. They were free already, but moving randomly. The voltage from the battery provides the energy to move them around the circuit.
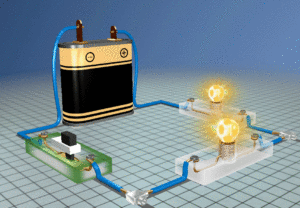
Figure 1 – The current is the same everywhere in this circuit, but the copper stays cool while the lamps get hot. Electrons have a hard time getting through the lamps and lose a lot of energy in collisions. This is converted to heat. They have an easy ride through copper, losing almost no energy at all.
Copper is unusual because it has more free electrons than most metals. This makes it a good conductor. Electrons can move through copper very freely. They hardly interact with copper atoms at all. Compare this with what happens in the lamps in the circuit. The lamp filaments are made of tungsten alloy. They have a high resistance to current and energy is transferred to the tungsten atoms as the electrons move through the filament. This energy heats up the filament to white heat.
Thermal conductivity
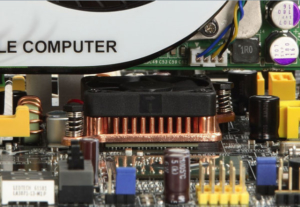
Copper has almost three times better heat conduction than aluminium. It can conduct heat rapidly from microprocessors that must be kept cool to work properly. Figures 2 and 3 show a specially designed ‘hedgehog’ heat sink with a fan that carries heat away from the processor chip.
(Courtesy of Enzotech.)
Figure 2 – Modern computer processors can be cooled by copper heat sinks with fans.These are often used in high power applications such as medical imaging.
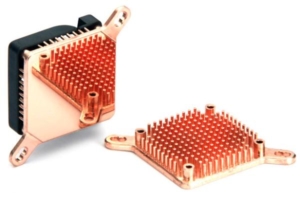
(Courtesy of Enzotech.)
(Courtesy of Codelco.)
Figure 3 – The shiny flat surface of the heat sink is bonded to the processor with conductive glue. The copper conducts heat into the pins, which have a large surface area. The fan helps transfer heat from the pins to the air.

(Courtesy of Falk.)Figure 4 – This copper pan uses stainless steel in the handle. Stainless steel is poor thermal conductor. It also uses a thin layer of stainless steel on the inside for a durable cooking surface.
The modern copper pan in Figure 4 is made from 2.5 mm thick copper with a very thin 0.2 mm inner layer of stainless steel and a stainless steel handle. Study the table below (click on it for a larger view) and see if you can explain why the pan is made that way. The table has a log scale on the x-axis so there is a 100,000 times difference in conductivity from the left side to the right side.
Forming and joining
Copper can be joined by soldering or the higher temperature process of brazing. It can be formed into complex shapes by bending.
This chart shows the thermal conductivity of common materials (Wikimedia Commons. Click to enlarge). The x-axis is on a powers of 10 scale (called a log scale).
On the left, the difference in conductivity between air and hollow fill fibre (as used in duvets) is very small. On the right, the difference between aluminium and copper is huge.
If this looks complicated, think how big the graph would need to be to get all that data on – about 1 km wide!
Questions and Activities
1. If a current of 1 amp is flowing in the circuit in Figure 1, how many free electrons flow though the wire per second?
2. What is the purpose of the pins on the heat sink in Figures 2 and 3?
3. In the thermal conductivity chart, the difference in conductivity between sandstone and lead looks about the same as the difference in conductivity between lead and copper. Is this really the case?
4. Copper is used in pans because it conducts heat well. Why does the stainless steel layer have very little effect on the conductivity?
5. Why is stainless steel used for the handle?
6. In the counter-current heat exchanger below, is the heat energy transferred from the clockwise flow to the anticlockwise flow or the other way around? How is the counter-current heat exchanger different from the heat exchanger in a whale’s flipper?

(Courtesy of Wieland-Werke AG.)
These coils are made from two copper pipes, one inside the other. Notice the double inlet and outlet ports. They make a counter-current heat exchanger. Two separate flows of liquid flow in opposite directions. The flow that starts hot transfers heat to the flow that starts cold. This clever design is found in nature in the flippers of whales and the legs of wading birds.
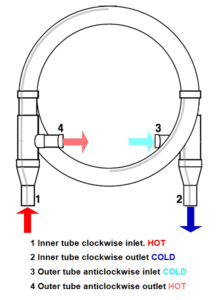
(Courtesy Wieland-Werke AG.)
This diagram of the counter-current heat exchanger coil above shows the two opposite flows. The diagram below shows how the same principle is used in a whale’s flipper.
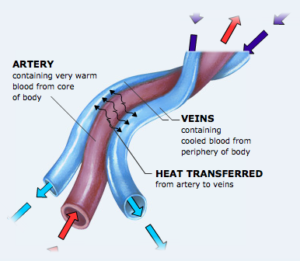
Heat exchange in a whale flipper. (Courtesy of Seaworld.)
In animals the tubes (veins and arteries) are side by side. In the copper heat exchanger they are one inside the other (coaxial).
The high thermal conductivity of copper allows heat energy to pass from the inner to the outer tube. It could equally well be connected the other way to pass heat from the outer to the inner tube.
The copper pipes can carry liquids or gases.
 |
 |

Comments
0 comments
Please sign in to leave a comment.