It’s hard to imagine a world without copper. We rely on copper for power, lighting, heating, communications, water supply and transport. Copper makes our homes, schools and businesses efficient, comfortable and decorative and it lasts for many years.
In this e-source, we’ll look at the ways of extracting copper from the ground.
Copper has a remarkable combination of properties. It is a good electrical and thermal conductor. It is ductile and it can prevent bacterial growth (see Copper: Properties and Applications).
As with all world resources, the supply of copper from the ground is limited. Ever since copper was first mined, it has been recycled. Today, nearly one third of demand for copper is met through recycling, helping to conserve the earth’s resources.
Copper is very well suited to being recycled because it can be remelted endlessly with no loss of properties. For example, old copper plumbing pipes, taps and car radiators are a major source of recycled copper. They can all be melted down and made into new products. Even the few grams of copper in your mobile phone is worth recovering.
Copper is an unreactive metal, it reacts only slowly with the atmosphere. This means that huge lumps of copper metal are found buried in the ground as nuggets. This is called native copper. The largest nugget of native copper ever found came from Minnesota, USA and weighed over 400 tonnes. Native copper isn’t mined because there is so little of it. Therefore it is of little commercial importance.
Mining Metals
Metals are often found as compounds in ores. An ore is a rock or mineral that has enough metal in it to make it worth extracting.
Mining copper
About 200 years ago the UK was an important world source of copper and tin and there were mines in Cornwall and Devon. These mines have now closed and today the biggest copper mines are in Chile (Escondida mine) and Indonesia (Grasberg mine). These produce many millions of tonnes of copper ore per year.
The main ores of copper are sulfides and oxides. From their chemical formulas you can calculate the percentage of copper in each mineral. However this is not the same as the percentage of copper in the ore as that also contains unwanted silicates and other minerals, called gangue, that will have to be separated.
| Mineral | Formula | % copper |
| Cuprite | Cu2O | See question 2 |
| Chalcocite | Cu2S | See question 2 |
| Bornite | Cu5FeS4 | 63 |
| Malachite | CuCO3Cu(OH)4 | 58 |
| Azurite | 2CuCO3Cu(OH)4 | 55 |
| Chalcopyrite | CuFeS2 | 35 |
Copper minerals and ore are found throughout the Earth’s crust. They occur in both sedimentary and igneous rocks. The outer 10 km of the crust contains 33 g of copper for every tonne of rock. This is not enough to make it commercially viable to extract the rock.
Copper mines are only set up where there is more than 5 kg of copper per tonne of rock (0.5% by mass). Ideally, the figure should be closer to 2%.
The world’s largest copper ore deposits are found in Chile on the west coast of South America. This is due to the volcanic activity that is part of the process that created the Andes mountain chain.
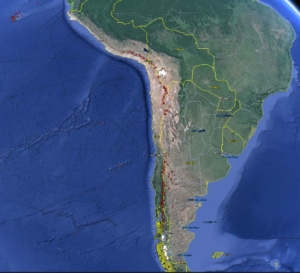
This is the west coast of South America, showing the line of volcanoes that stretches along the whole west coast of the continent. They are caused by subduction of the Nazca tectonic plate under the continent. Click to enlarge image. (Courtesy of Smithsonian/Google Earth.)
These processes created the Andes mountain chain that you can see behind the picture of El Teniente (below). Millions of years of volcanism and mountain building have created concentrations of copper and other precious metals. They also cause earthquakes, which are a constant threat for mines.

This the processing plant at one of Chile’s largest copper mines, El Teniente. (Courtesy of Codelco.)
The British Geological Survey and Smithsonian Institute have sites and apps that will show you the current and historical volcanic activity. Volcanic copper deposits are called porphyry copper.
Web search hints: Porphyry copper – Nazca subduction – Global volcanism smithsonian
Copper can be extracted from its ore by:
- Underground: sinking a vertical shaft into the Earth to an appropriate depth and driving horizontal tunnels into the ore.
- Open pit: 90% of ore is mined by this method. Ores near the surface can be quarried after removal of the surface layers. The ore is treated with dilute sulfuric acid. This trickles slowly through the ore dissolving copper to form copper sulfate. The copper is recovered by electrolytic refining. Advantages of this process are: See for further information on the leaching process.

Escondida in Chile is the biggest copper producer in the world, accounting for 5% of global production. (Courtesy of BHP Billiton.)

Copper ore is loaded into trucks. In some mines these trucks have no driver. (Courtesy of Codelco.)

This truck carrying copper ore has no driver. It is guided by a GPS system. Can you see the GPS antenna? (Courtesy of Codelco.)
Processing Stages
Stage 1: Crushing and grinding
The ore from the mine contains about 2% copper. To get at the copper the first stage is crushing in huge cylindrical ball mills.

Ball mills crush the ore into a fine powder. (Courtesy of Rio Tinto.)
Stage 2: Froth flotation
The powdered ore is mixed with a special paraffin oil which makes the copper mineral particles water repellent. It is then fed into a bath of water containing a foaming agent which produces a kind of bubble bath.

This is a rare chance to see a mill and flotation building before the roof is built. (Courtesy of Codelco.)

Froth flotation tanks. (Courtesy of Codelco.)
When jets of air are forced up through the bath, the water repellent copper mineral particles are picked up by the bubbles of foam. They float to the surface making a froth. The unwanted waste rock (gangue) falls to the bottom and is removed.
The froth is skimmed off the surface and the enriched ore (mainly the copper mineral) is taken away for roasting. The mixture of water, foaming agent and paraffin is recycled.
After this stage the enriched ore now contains about 25% copper by mass and is called copper concentrate. It is valuable enough to ship to other plants and other countries for processing. For example China, Germany and Japan are major copper producers that use concentrate from around the world.
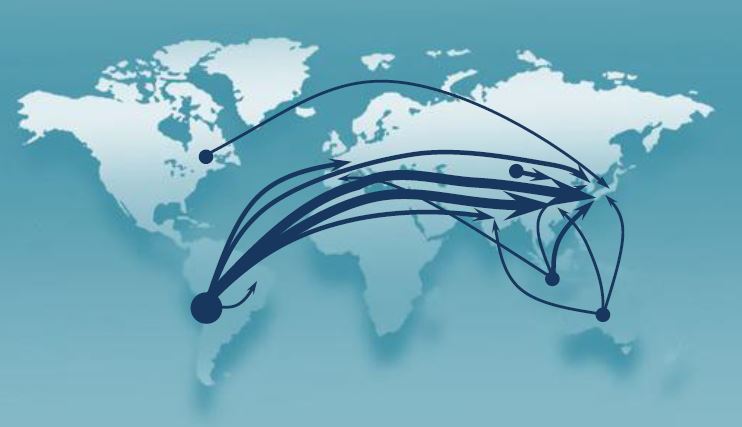
This diagram shows the international trade flow in copper concentrate.The flow is from major mining countries to major industrial countries (Source: International Copper Study Group). The total trade in 2013 was over 25 million tonnes. Chile exported 8.4 million tonnes.
Stage 3: Roasting
At this stage of the process, the chemical reactions begin. They convert the copper minerals into copper metal.
We can illustrate the types of process using the example of chalcopyrite – CuFeS2. From the formula, it is clear that iron and sulfur have to be removed in order to produce copper.
The roasting process:
- changes some of the CuFeS2 to copper oxide
- removes some of the sulfur as sulfur dioxide
This is done by heating the concentrated ore from froth flotation. It is heated to between 500 °C and 700 °C in air. The product from the roaster is called calcine. It is a solid mixture of oxides, sulfides and sulfates. One reaction that takes place is:
2CuFeS2(s) + 3O2(g) → 2FeO(s) + 2CuS(s) + 2SO2(g)
As SO2 is hazardous, it has to be removed from the gases released into the environment. One way of solving the problem is to convert it to sulfuric acid. This is either sold for industrial use or used to extract oxide ores of copper by leaching.
Stage 4: Smelting with fluxes
The calcine is heated to over 1200 °C with fluxes such as silica and limestone. The calcine melts and reacts with the fluxes. Some impurities form a slag (such as FeO.SiO2), which floats on the surface of the liquid (like oil on water) and is easily removed.

(Courtesy of Xstrata.)
The smelter for sulfide ores produces sulfur dioxide gas. This is scrubbed from the flue gases to make sulfuric acid for leaching copper from oxide ores. Scrubbing sulfur dioxide also protects the environment.
For example:
FeO(s) + SiO2(s)→ FeO.SiO2 (slag)
This is very similar to the removal of impurities in the blast furnace. The liquid left is a mixture of copper sulfides and iron sulfides. It is called a matte.
Stage 5: Conversion of matte to copper blister
The liquid matte is oxidised with air to form blister copper in a converter.

Blister copper from the smelter is poured into the anode casting moulds. The anodes are cast into slabs about 1 metre square with arms at the top corners for lifting. (Courtesy of Codelco.)
The reactions are:
a) Elimination of iron sulfide by oxidation to iron oxide which forms a slag:
2FeS(l) + 3O2 + SiO2(l) → 2FeO.SiO2 + 2SO2 (g)
air flux slag gas
b) Formation of blister copper by reduction of copper sulfide:
Cu2S(l) + O2(g) → 2Cu(l) + SO2
air blister copper gas
Stage 6: Anode casting
The blister copper produced by this process is 99% pure copper. The name ‘blister’ copper comes from the fact that this final process produces bubbles of sulfur dioxide on the surface of the copper. The blister copper is cast into anodes ready for electrolytic refining.

The anode casting turntable moves slowly round. The anodes can be lifted out at the far side. (Courtesy of Codelco.)
This huge wheel is used to cast the anodes. Molten copper is poured into a mould and the wheel is turned for the next mould. Meanwhile, the molten copper cools and solidifies into anodes over a metre tall. The mould includes short supporting arms used to lift and suspend the anodes.
Lifting the solidified anodes into cooling tanks. (Courtesy of Codelco.)
Stage 7: Electrolytic refining
The blister copper is already virtually pure (in excess of 99% copper). But for today’s market, this is not really pure enough! It is purified further using electrolysis. This is known as electrolytic refining.
The blister copper is cast into large slabs which will be used as the anodes in the electrolysis apparatus. The electrolytic refining of copper produces the high quality, high purity copper required by industry.
Even the best chemical method cannot remove all the impurities from the copper, but with electrolytic refining it is possible to produce 99.99% pure copper.

Copper cathodes are hung between anodes. (Courtesy of Aurubis.)
Copper cathodes are hung between anodes. Can you recognise the shape of the suspension arms on the anodes from the picture of anode casting? Copper atoms on the anode lose electrons to become Cu2+ ions in the electrolyte. At the cathode the copper ions take back two electrons to return to being copper atoms. It may seem that the whole process achieves nothing: Cu → Cu2+ → Cu .
The purification happens because the other metals in the anode (impurities) will not dissolve in the electrolyte solution. They fall to the bottom of the tank. The cathodes are 99.99% pure copper.
The blister copper anodes are immersed in an electrolyte containing copper sulfate and sulfuric acid. Pure copper cathodes are arranged between the blister copper anodes and a current of over 200A passes through the solution. This is driven by a low voltage of about 1.3V and so the process is safe.
What happens in electrolysis?
Under these conditions, copper atoms dissolve from the impure anode to form copper ions. These migrate towards the cathodes where they are deposited back as pure copper atoms.
At the anode: Cu(s) → Cu2+(aq) + 2e-
At the cathode: Cu2+(aq) + 2e- → Cu(s)
This is the same reaction as the one you may have tried to perform in the school laboratory.
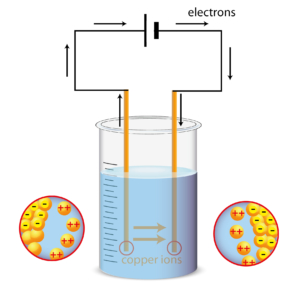
Laboratory electrolysis. In copper refining many anodes and cathodes are suspended alternately. All the cathodes are connected to the negative terminal and all the anodes to the positive terminal.
Copper atoms on the anode surface lose two electrons becoming soluble Cu2+ ions.
Cu2+ ions have a positive charge. They find themselves in an electric field between the electrodes.
Being positive, they move to the negative cathode where they pick up two electrons and go back to being metallic atoms with no charge.
In an electric circuit, electron flow goes from negative to positive so the process transports electrons from the cathode to the anode.
Gradually, the anode is eroded and the cathode grows. Insoluble impurities in the anode fall to the bottom as a sludge.
What happens to the impurities?
Gold, silver, platinum and tin are insoluble in this electrolyte and so do not deposit on the cathode. They form a valuable ‘sludge’ that collects under the anodes.
Soluble impurities of iron and nickel dissolve in the electrolyte, which has to be continually purified to prevent excessive deposition onto the cathodes, which would reduce the purity of the copper.
Recently, stainless steel cathodes have replaced copper cathodes. Identical chemical reactions take place. Periodically, the cathodes are removed and pure copper is scraped off.

Cathode copper is remelted and cast into billets ready for turning into copper products. (Courtesy of Aurubis.)
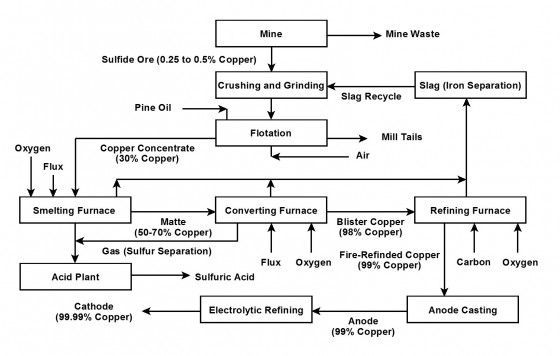
This flow diagram summarises the sulfide ore processing stages. Find the two furnaces that send sulfur dioxide gas to the acid plant.
Questions and Activities
- Place these products in the extraction process in ascending order of copper content. Use the flow chart as a guide:
Matte
Ore
Cathode copper
Blister
Anode copper
Copper concentrate
Fire refined copper - Use a periodic table to find the atomic masses of the elements in cuprite and chalcocite. Then work out the percentage of copper in each mineral. Don’t forget to double the copper mass as it is Cu2.
- Which of the minerals in the table are sulfides?
- Why is sulfur dioxide scrubbed from the smelter flue gases?
 |
 |
Comments
0 comments
Please sign in to leave a comment.