<< Back to Copper Recycling and Sustainability Part 3 (16-18)
1. In Chart 2, why is the ratio of tonnage of primary and secondary sources not the same as the 59% to 41% of sources of copper?
Recycled copper is already refined. The primary tonnage is for copper concentrate, which only contains about 30% copper.
2. Why is bronze used to make ship propellors? (Think about manufacturing and use at sea.)
Bronze is ideal for casting into complex shapes in a single pour. It is extremely tough so that its central bearing will survive billions of revolutions. Bronze is naturally anti-fouling, so the propellor stays clean. Bronze is very strong. The propellor can be designed with thin blades for efficiency. The scrap value of an old propellor is very high. Bronze will not corrode in sea water.
3. In Chart 1, which form of copper extraction has grown from zero in the 1960s to almost 4 million tonnes in 2013?
Solvent extraction. Sulfuric acid is poured through a heap of copper ore. The acid reacts with the copper to form copper sulfate solution. The copper sulfate solution is then used as the electrolyte in electrolytic refining.
The last part of the process is called electrowinning. Hence the full name of Solvent Extraction – Electrowinning (SX-EW). The smelting process is by-passed. The process goes direct from crushed ore to electrolytic refining.
SX-EW has two big benefits for the copper mining industry. First, it uses less energy than froth flotation and smelting. Second, it is effective on lower grade ores that would be uneconomic for smelting.
SX-EW is predicted to overtake pyro-processing in the future. However, pyroprocessing of sulfide ores produces the sulfuric acid needed for solvent extraction. The industry needs to balance the two methods.
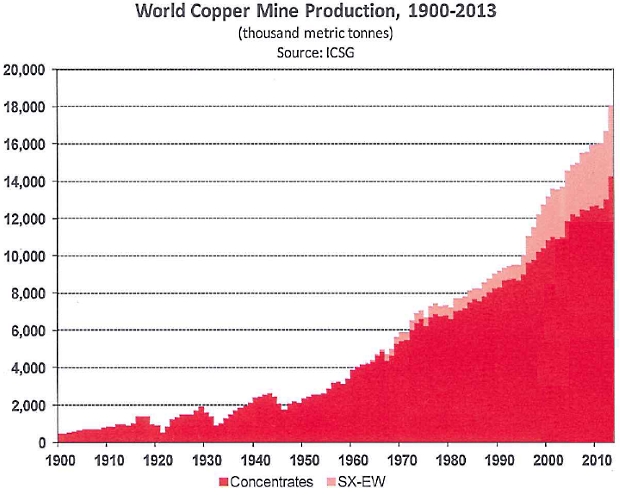
Chart 1: (Courtesy of ICSG.)
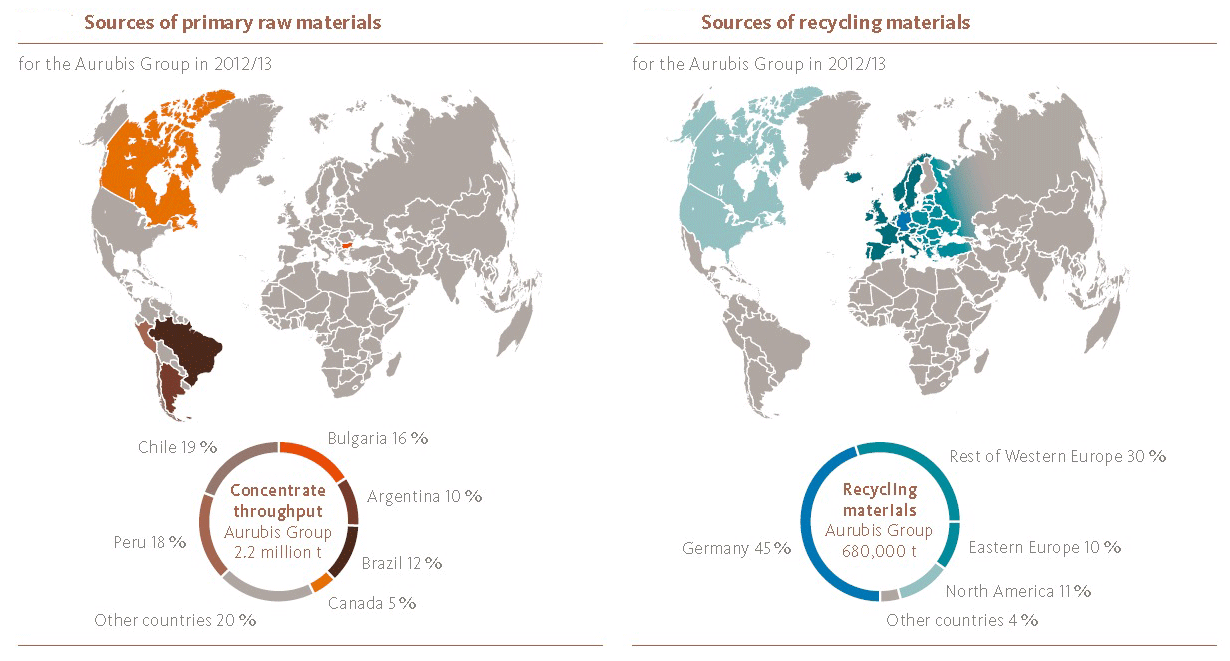
Chart 2: (Courtesy of Aurubis.)
The pictures below show the four main steps of Solvent Extraction and Electrowinning (SX-EW)

Sulfuric acid is poured into a heap of crushed ore. You can see the outlet pond in the distance. Note the acres of waterproof and acid proof membrane fabric. (Courtesy of Red Tiger Mining.)


The solvent extraction plant. Blue copper sulfate solution is fed to the electrowinning tanks. (Courtesy of Red Tiger Mining.)

Copper is deposited onto steel cathode plates and is stripped off when it is thick enough. Copper ions in the solution are now copper atoms. Hydrogen ions replace them, turning the electrolyte back to sulfuric acid.
Comments
0 comments
Please sign in to leave a comment.