Copper and copper alloys offer a suite of infinitely recyclable materials providing many property combinations suited to a wide range of applications that facilitate and enhance our daily lives.
Copper’s performance can be expanded to suit many industrial applications by alloying: making a solid material out of two or more different metals. Good electrical and thermal conductivity, strength, ductility and excellent corrosion resistance are just some of the properties that copper and its alloys offer. Copper alloys are grouped into families, based on their composition.
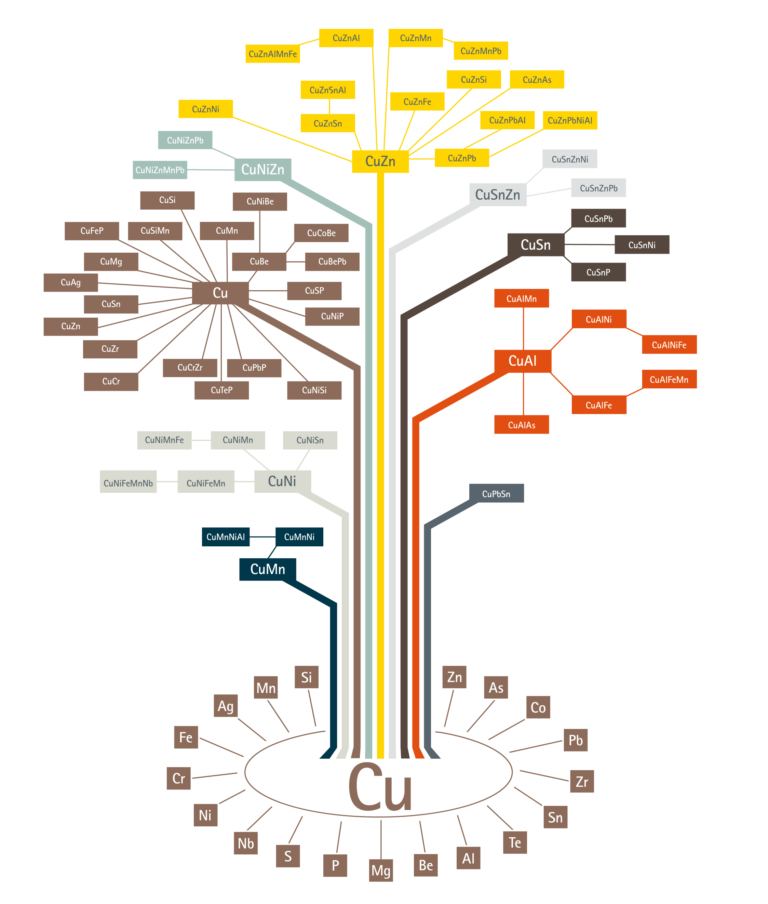
There are more than 400 copper alloys, each with a unique combination of properties, to suit many applications, manufacturing processes and environments.
Pure copper has the best electrical and thermal conductivity of any commercial metal. Today, over half of the copper produced is used in electrical and electronic applications and this leads to a convenient classification of the types of copper into electrical (high conductivity) and non-electrical (engineering). Go to the Coppers section.
Copper forms alloys more freely than most metals and with a wide range of alloying elements to produce the following alloys:
Brass is the generic term for a range of copper-zinc alloys with differing combinations of properties, including strength, machinability, ductility, wear-resistance, hardness, colour, hygienic, electrical and thermal conductivity, and corrosion-resistance. Go to the Brasses section.
Bronze alloys are made from copper and tin, and were the first to be developed, about four thousand years ago. They were so important that they led to a period in time being named the Bronze Age. Go to the Bronzes section.
Gunmetals are alloys of copper with tin, zinc and lead and have been used for at least 2000 years due to their ease of casting and good strength and corrosion resistance. Go to the Gunmetals section.
Copper-nickel alloys have excellent resistance to marine corrosion, high thermal conductivity and low susceptibility to attachment of marine macro-organisms. The addition of nickel to copper improves strength and corrosion resistance, but good ductility is retained. Go to the Copper-nickels section.
Nickel silver alloys are made from copper, nickel and zinc, and can be regarded as special brasses. They have an attractive silvery appearance rather than the typical brassy colour. Go to the Nickel silvers section.
Beryllium-copper is the hardest and strongest of any copper alloy, in the fully heat treated and cold worked condition. It is similar in mechanical properties to many high strength alloy steels but, compared to steels, it has better corrosion resistance. Go to the Beryllium copper section.
Copper is a metal that is extracted from the earth, is essential to the development of all forms of life and has been vital in the progress of civilisation, contributing to both social and technological development for more than 10,000 years.
The valuable properties of copper which were evident at the dawn of civilisation were an attractive colour, excellent ductility and malleability, and a capability of being hardened by working. In modern times, further properties have been appreciated and exploited across a wide range of applications: high thermal and electrical conductivities, excellent corrosion and hygienic properties.
Today, copper and copper alloys make a significant contribution to the latest developments in renewable energy, information and communication technology, architecture, health and sanitation. The production and use of copper are also vital parts of our economy. Copper is simply essential for life.
Comments
0 comments
Please sign in to leave a comment.